On January 18, the U.S. reached 249.4 million persons (75.1% of the total U.S. population) having received at least one dose of a COVID-19 vaccine and 209.3 million persons (63.0% of the total U.S. population) being fully vaccinated (i.e., having received either one shot of the Johnson & Johnson/Janssen vaccine or two shots of either the mRNA Pfizer/BioNTech or Moderna vaccines). Jurisdictions with the highest percentages of persons 5 years of age and older being fully vaccinated include Vermont (82.5%), Rhode Island (82.1%), Puerto Rico (81%), Connecticut (79.9%), and Massachusetts (79.8%). States with the lowest include Wyoming (51.3%), Mississippi (52.3%), Alabama (54.5%), Louisiana (54.5%), and Arkansas (55.4%).
Delta and Omicron Variants
Thus far, there is consensus that Omicron is the most transmissible variant yet. According to the CDC, Omicron is now the dominant strain in the United States, making up 99.5% of all new cases as of January 15 (the remainder are Delta). Its increased transmissibility may be due to how much more effectively it replicates in the upper respiratory tract.
It has been challenging for scientists to determine if or for whom Omicron produces less severe disease. There may be differences in the populations in which the variant spreads (e.g., Omicron may have first spread among younger, healthier individuals who are at less risk of being hospitalized in the first place). Another issue might be that not enough time has passed – hospitalization rates typically lag a few weeks behind cases. Even if Omicron appears to be slightly less severe (e.g., causing lower rates of hospitalization), it is also extremely transmissible, causing hospital systems to still be inundated with huge numbers of patients and/or short on staff as they too become sick.
It will also take time to determine how well, if at all, existing immunity from prior infections and/or vaccination protects against Omicron. Early data from South Africa suggests that Omicron infects those with existing immunity at rates higher than with previous variants. As with Delta, it is uncertain whether Omicron is better at evading immune defenses or if people’s protection is waning to the point that they are more vulnerable.
The Omicron trajectory in other parts of the world may not translate to the U.S. In South Africa, persons infected by Omicron were more likely to be younger and have fewer comorbidities that put them at increased risk of adverse health outcomes. As another example, compared to other high-income countries in Europe, the U.S. lags significantly in the proportion of fully-vaccinated and boosted individuals, which may also affect the Omicron trajectory here.
It is impossible to definitively predict how the U.S. and the rest of the world will fare following this current Omicron wave. Some public health experts believe that Omicron will infect and therefore provide infection-induced immunity for enough of the population to protect against future variants. Others predict that any lull in infections could be temporary either because another more virulent and/or transmissible variant could arise or protection from prior infections and/or vaccines could wane.
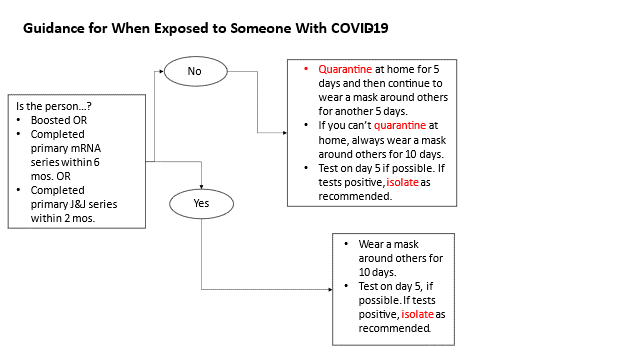
Updates to CDC Guidance on Isolation and Quarantining
In response to the exponential surge of Omicron and concern about significant societal disruptions, the CDC updated and shortened the recommended isolation and quarantine period. “Isolation” is defined as the period in which a person who has COVID-19 should avoid others to reduce the risk of transmission. “Quarantine” is used to describe the period when a person who has been exposed to COVID-19, but may not actually have been infected, should avoid others. The following charts summarize the latest recommendations from CDC:
What to Do If You Test Positive
- Isolate (i.e., stay at home and away from others) for 5 days. If person has symptoms, Day 0 of isolation is the first day symptoms occur. If person has no symptoms or is asymptomatic, Day 0 is the day that they first test positive for COVID-19.
- If have no symptoms or symptoms are resolving after 5 days, can leave isolation and wear a mask around others for 5 more days.
- If still has symptoms after 5 days, stay at home and away from others until symptoms are gone.
Since their release, these updated guidelines have faced mixed reactions from public health experts, politicians, and the public. Many have felt that the updated guidance was confusing and difficult to interpret for the average person. Among public health experts specifically, many disagreed with the CDC’s decision to not include a stronger recommendation that persons receive at least one negative rapid antigen test result before leaving isolation. While they agreed that the bulk of transmission occurs in the first five days of an infection, these experts argue that some persons may still be infectious beyond the first five days with or without symptoms. A negative rapid antigen test would further provide assurance that the person is indeed no longer able to transmit to others and it is therefore safe for them to leave isolation.
Boosters
As of January 18, 81.0 million persons in the U.S. have received a booster (i.e., a third dose following an mRNA primary two-dose series or a second dose following a Johnson & Johnson/Janssen primary one-dose series). This represents 38.7% of those who are fully vaccinated, including 41.8% of those aged 18 years and older, 53.4% of those 50 years and older, and 62.2% of those 65 years and older. States with the highest rates of boosters administered among their fully vaccinated populations include Vermont (54.5%), Minnesota (50.8%), Wisconsin (49.5%), Maine (49.4%), and Rhode Island (49%).
On January 5, the Centers for Disease Control & Prevention (CDC) endorsed the expansion of booster eligibility to persons 12 years and older who received a primary two-dose BioNTech/Pfizer vaccine series. At that time, the CDC also shortened the period between the second shot in a person’s primary series and their booster to five months for the Pfizer/BioNTech vaccine. On January 7, the CDC similarly shortened the period for those who received the Moderna vaccine primary series. The following table summarizes when and what kind of booster different individuals are now eligible for:
| Primary Vaccine Series | Groups Eligible to Receive Booster | When to Receive Booster | Booster Doses Can Receive |
|---|---|---|---|
| Johnson & Johnson / Janssen | Those 18 years and older | At least two months after first dose | • 1 dose Johnson & Johnson/Janssen • 1 full dose of Pfizer/BioNTech • 1 half dose of Moderna |
| Moderna | Those 18 years and older | At least six months after second dose * | • 1 dose Johnson & Johnson/Janssen • 1 full dose of Pfizer/BioNTech • 1 half dose of Moderna * |
| Pfizer/BioNTech | Those 12 years and older | At least give months after second dose * | • 1 dose Johnson & Johnson/Janssen • 1 full dose of Pfizer/BioNTech ** • 1 half dose of Moderna * |
* Immunocompromised individuals aged 5 years of age and older (the vast majority of whom received a two-dose primary mRNA series) are recommended to receive a third mRNA vaccine dose 28 days after their second shot. If they are receiving a third Moderna shot, it should be a full-sized dose. At least five months after their third shot, moderately or severely immunocompromised individuals may receive a fourth mRNA dose.
** Adolescents aged 12 – 17 years of age can only receive a third dose of the Pfizer/BioNTech vaccine as a booster. To date, the Pfizer/BioNTech vaccine is the only one to be authorized for this age group as either a primary series or booster.* Immunocompromised individuals aged 5 years of age and older (the vast majority of whom received a two-dose primary mRNA series) are recommended to receive a third mRNA vaccine dose 28 days after their second shot. If they are receiving a third Moderna shot, it should be a full-sized dose. At least five months after their third shot, moderately or severely immunocompromised individuals may receive a fourth mRNA dose.
Among booster doses administered nationally, the majority (44 million or 54.2%) have been Pfizer/BioNTech followed by Moderna (35.8 million or 44.2%) and Johnson & Johnson/Janssen (1.2 million or 1.5%). Those who received either a Pfizer/BioNTech or Moderna primary series largely stuck to the same vaccine type for their booster (92.8% and 92.4%, respectively). Individuals that initially received a single shot of the Johnson & Johnson/Janssen vaccine were roughly split between each of the three authorized vaccines with 33.3% receiving a Pfizer/BioNTech booster, 41.7% receiving a Moderna booster, and 25% receiving a second shot of Johnson & Johnson/Janssen. Of note, since December 16, the CDC recommends individuals receive either of the two available mRNA vaccines over the Johnson & Johnson/Janssen vaccine as a primary series or booster.
Scientists and public health officials continue to analyze and release real-world data regarding the short- and long-term efficacy of the vaccine boosters, including against the Omicron variant. Preliminary studies by Pfizer/BioNTech and Moderna indicate that people who only received two shots of either vaccine had antibodies that were far less effective in neutralizing Omicron. This likely leaves these individuals much less protected against infection than with previous variants. Both boosters were able to sharply increase the number of neutralizing antibodies that could block Omicron, bringing the level of protection against infection in line with two-shots against the Delta variant. As a result, while the CDC has not yet altered their definition of “fully-vaccinated,” they have shifted their guidance by recommending that individuals “stay up to date” on their COVID-19 vaccines by receiving recommended boosters.
Limited real-world data is available on how much and how long a first booster dose will protect against infection, hospitalization, and death. In Israel, the first nation to launch a robust booster campaign, high-risk groups (e.g., moderately and severely immunocompromised individuals), healthcare workers, and people over 60 years of age are eligible to receive a fourth dose at least four months following their third. Early data suggests that protection against infection wanes three months following a third dose. A fourth dose produces fivefold increases in antibody levels with a similar safety profile as with third shots.
Meanwhile, Pfizer/BioNTech and Moderna have both announced plans to study the effectiveness of Omicron-specific vaccines. Pfizer/BioNTech has stated that an Omicron vaccine would be ready by March 2022 and that they are already producing doses at-risk (i.e., producing doses of a vaccine that may not actually ever be purchased or used). Moderna, meanwhile, is preparing its own Omicron vaccine for fall 2022. It remains unclear if an Omicron-specific vaccine is necessary and/or if a new variant would emerge before the Omicron-specific vaccines could be available globally.
COVID-19 Vaccines for Children
Following the winter holidays and the rise in Omicron prevalence in the U.S., COVID-19 cases and hospitalizations have risen to record levels among children and adolescents. At the beginning of January, hospitalizations were 48% higher among children than the prior month. As a result of students, teachers, and staff increasingly exposed to or sick with COVID-19 and/or other respiratory diseases (e.g., the flu), school closures and other disruptions are also increasing. Meanwhile, the roll-out of COVID-19 vaccines for children, particularly those under 12, has largely stalled with very low and uneven uptake.
The pediatric vaccine roll-out was considered fully up and running as of Monday, November 8. As of January 12, 27% of 5 – 11 years old children are estimated to have received one or more vaccine doses while 18% are considered fully vaccinated. While vaccination rates among these children increased significantly after the CDC first recommended it on November 2, they have steadily decreased since the peak on November 16. There have also been state-by-state variations, with as much as a forty-five percent difference between the state with the highest proportion of fully-vaccinated 5 – 11 years old children (Massachusetts, 49.5%) and lowest ranked states (Alabama, 4.7%). For comparison, about half of parents of adolescents aged 12 – 17 years say that their child has received at least one dose.
The FDA and CDC have continued to closely monitor children who have received the Pfizer/BioNTech vaccine via the Vaccine Adverse Event Reporting System (VAERS) and the V-safe After Vaccination Health Checker. The CDC has released data on the safety of the vaccines among children 5 – 11 years of age which demonstrate that they are incredibly safe with very few serious adverse events reported in this age group. In terms of efficacy, the vaccine is reported to have 92% efficacy against COVID-19 positive cases among adolescents based on real-world data. Further, the vaccine is highly protective against multisystem inflammatory syndrome in children (MIS-C) in this same age group. While additional real-world efficacy data will eventually be available for children 5 – 11 years of age, it is anticipated that the vaccine will remain protective particularly against severe disease. Booster shots for these children will also be considered by the FDA and CDC at some point in the coming weeks. Already, third shots are authorized for children aged 5 – 11 years of age with moderate or severe immunocompromise.
Future Considerations
In the weeks ahead, members of the attorney general community should monitor and be aware of the following possible key events related to the vaccines’ rollout:
- Further updated guidance released by the CDC and state/local health departments regarding the need for non-medical interventions (e.g., mask wearing, social distancing) by fully vaccinated, boosted, and unvaccinated individuals generally and within specific settings. For example, the CDC has formally endorsed a “test to stay” strategy in schools based on the results of real-world studies performed in Lake County, Illinois and Los Angeles County, California.
- Potential changes to the definitions of “fully vaccinated” to include a booster dose and impacts that may have on vaccine requirements.
- Potential grant by FDA of a EUA for Pfizer/BioNTech vaccine for children under age 5 years of age.
- Additional information on the (1) transmissibility, (2) disease severity, and (3) potential for immune escape of the Omicron variant.
- Release of additional data regarding the safety and efficacy of mixing and matching vaccines for purposes of boosters.
- Increased availability of two oral antiviral treatments for COVID-19 from Merck and Pfizer that both received EUAs from the FDA in December 2021. Also, additional data on the efficacy of existing and future treatment options against Omicron.
- Additional vaccine manufacturers possibly seeking authorization in the U.S., including the two-dose AstraZeneca/Oxford University and Novavax.
In 2022, NAAG will continue to provide informational updates and training opportunities to the attorney general community as COVID-19 vaccine distribution and related legal issues evolve. For more information on NAAG’s response to the COVID-19 pandemic, visit NAAG’s public health-related updates.




